Updated: 30 Mar Sept 2015
Alco-Vapor engine added




Alcohol Engines |
Updated: 30 Mar Sept 2015 |
Alcohol vapourises easily, but that does not mean it is more efficient than water as a working fluid. Quite the reverse. See: Carnot's Law on the thermodynamics page.
AN ALCOHOL VAPOUR ENGINE DEMONSTRATION
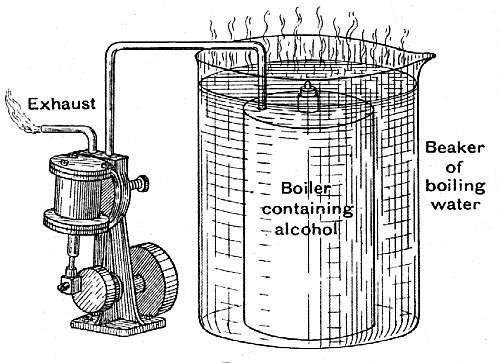 | Left: A toy steam-engine driven by alcohol vapour, with the exhaust combusting.
The author points that the alcohol vapour exhaust can be lit, though it would be wise to make sure that all the air has been driven out of the plumbing before trying it. This underlines that a non-condensing (total-loss) alcohol vapour engine is a grotesquely inefficient and expensive proposition. I hasten to add that the author was not claiming otherwise, but goes on to deal with the concept of Carnot efficiency. |
THE HISTORY OF ALCOHOL VAPOUR ENGINES
The French journal Nature, (21 June, 1890) has this to say: "Artwright proposed and constructed, in 1797, an alcohol-vapour machine which worked well enough, but which did not survive, very probably because of the very imperfect construction of the time; substantial leaks of a relatively costly vapour would have made the system very uneconomic."
I have long suspected that "Artwright" should be "Arkwright", but actually it looks as though the name was Cartwright; the entry below refers to Dr Edmund Cartwright, but it only says he suggested an alcohol engine, not that he actually constructed one.
 |  | Left: The entry on alcohol engines from Knight's Dictionary
| ||||
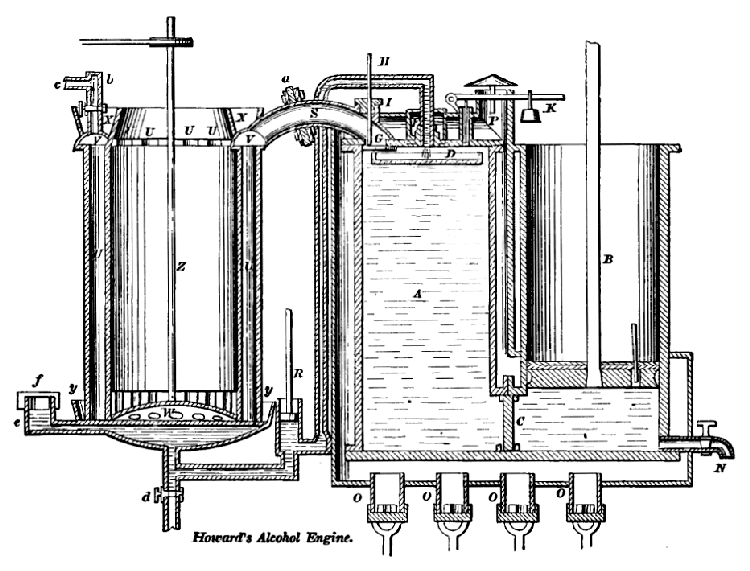
Above: Howard's alcohol engine: 1825
Howard appears to have been fully aware of the need to minimise the losses through leakage of an expensive working medium. He used hot oil as a liquid piston. (Early experiments seem to have beeen done with mercury, but the cost of this for a large engine would have been crippling. Breathing the mercury vapour would probably have been crippling too) In the diagram above a flat dish D can be seen floating on the hot oil A in the middle cylinder. The oil was kept hot by the four Argand burners underneath. Alcohol was sprayed into the hot dish by the narrow pipe just above it, propelled by pump R, and flashed into vapour. This pushed down the oil and pushed up the piston in cylinder B on the right.
This engine, with its complexity and its interesting combination of alcohol and hot oil, seems wholly impractical. No wonder that according to Knight, it "...wearied out the patience or the means of its inventor...".
BINARY VAPOUR ENGINES
A binary vapour engine boils a mixture of liquids, usually water and some kind of alcohol. It doesn't appear to be a good idea, but that has not stopped a few people from trying it.
US NAVY EXPERIMENTS WITH WATER/METHANOL
In 1885 a commission of US Navy engineers tried out a mixture of water and 15% wood alcohol, Methanol was presumably chosen for its low boiling point, though this is fallacious. (see below) Perhaps they thought its toxicity would deter the crew from drinking it. Historically this approach has been of questionable effectiveness.
The account reproduced here is taken from the British journal Engineering for 9th Jan, 1885. Wood alcohol is methanol (methyl alcohol)
The major problem with the later large-scale test seems to have been leakage; not good when the vapour leaking is toxic, inflammable and relatively expensive. Whether methanol really does leak out more easily than steam I have not so far been able to determine; presumably it depends on the size of the vapour molecule.
To learn more about constant boiling mixtures see here. (external site)
OLFELDT'S ALCO-VAPOR ENGINES: WATER/ETHANOL
Frank W Ofeldt is most closely associated with the naphtha launch, but when he left the Gas Engine and Power Company he produced the Alco-Vapor power plant for launches. This boiled a mixture of water and alcohol, not because the lower boiling-point of alcohol would make the engine more efficient (the exact opposite is true) but in not very impressive attempt to evade the USA regulations that required every steam launch to carry a licensed steam engineer. The naphtha launch was the first approach to this, and it seems to have worked in evading the law, at the cost of the inherent hazards in boiling petrol.
Nowhere have I found confirmation that 'alcohol' in this context means 'ethanol', but given the problems that were experienced when methanol was used, as described above, it seems extremely likely. In the journal MotorBoating (Nov 1942, p46-48) the proportion used was described as "a merely nominal amount of alcohol" and if the question had arisen, it is hard to see how Ofeldt could have argued at all convincingly that he was not building steam engines.
The convenient layout with the fuel at one end and the power-plant at the other was retained from the naptha launches, but the ingenious principle of burning the working fluid as fuel was abandoned, probably because of the low calorific value of alcohol when it burns. Heat was provided by burning what the patent calls "head-light oil".
Frank W. Ofeldt's alco-vapor patent was US 551,226, granted 10th December 1895, and assigned to The Marine Vapor Engine Company.
This is taken from page 4 of the Marine Vapor Engine Company's 1897 catalogue:
"As we use alcohol expansively for power, it may be asked, wherein does it differ and in what respect is it better than the use of naphtha? In answer to the first query, we wil state that the alcohol has the peculiar property of expanding a greater number of times and at a lower temperature than naphtha. John W Nystrom, Civil Engineer, a recognised authority, gives the boiling point of naphtha at 320 degF (160 degC), water at 220 degF (100 degC), and alcohol at 173 degF (78 degC). So it will appear quite obvious that should we begin to create pressure at 173 degF, by the time we have increased the temperature to the point of boiling water we have fifteen pounds, and before we reach the boiling point of naphtha we have a pressure of 100 pounds."
"In fact, owing to the low boiling point of the alcohol, it is not necessary in starting the Alco engine to labouriously work the liquid out of the cylinders by turning the engine by hand as in the case of the naphtha, for immediately we have 25 lbs pressure- our engine starts without aid, and owing to the small amount of heat required one can fearlessly place their hand upon the casing of the engine when running at 100 ilbs pressure. But our intelligent critic may say, admitting all the advantage you claim, is not alcohol dangerous? Our answer isno; for unlike naphtha, it combines with water in all proportions, and should any be allowed to escape, the fact that there is always a small quantity of water in the bilge eliminates any likelihood of fire. This could be demonstrated by the simple experiment of takig spoonful of alcohol, igniting it, and then pouring it into a glass of water. The flame will be immediately extinguished, while it is a well-known fact that naphtha burns more intensely upon the surface of the water than elsewhere."
"As we carry but one-tenth as much alcohol as is usually carried of naphtha by naphtha or gasoline launches, and use the same over and over with but little attention during the entire season, the convenience and safety of our system will be readily seen and appreciated."
(I inserted the Celsius temperatures)
I had my doubts about John W Nystrom, but there he is in Wikipedia. Unfortunately he seems to have been a bit of a nutter: Nystrom is best known for his proposal in 1859 to switch from base-10 to base-16 number systems. By 1875 he had changed his mind and was advocating base-12. This sort of thing does not inspire confidence. At first I assumed he was called on as a consultant as a result of his book A new treatise on steam engineering, physical properties of permanent gases, and of different kinds of vapor (Philadelphia: London: J.B. Lippincott & Co. 1876) But... Wikipedia has his dates as 1825–1885 so he did not make old bones. More to the point, he had been dead for 12 years when The Marine Vapor Engine Company were quoting him as an expert.
Olfeldt's personal history can be found on the page devoted to the The Petrol-Vapour Motors.
On the return stroke the alcohol vapour was pushed into the condenser on the left via pipe S. The condenser tubes U were wrapped in wet flannel, with air blown over them by a fan mounted on vertical shaft Z.
Note that the board of US Navy engineers had grave (and entirely justified) doubts about the thermodynamics of low-boiling-point liquids as working fluids. (Methanol boils at 64.7 degC) The US Navy has not always distinguished itself in this field.


Above: An experiment with a binary working fluid- in this case water and methanol.
Another pitfall was clearly the tendency of this binary mixture to change its composition when boiled, so that the condensed liquid in the hotwell was much richer in methanol than the liquid in the boiler. For an ethanol-water mixture, the vapour would not boil off in the same proportions as in the liquid in the boiler unless the methanol concentration was a lot higher than 15%; ethanol-water forms a constant-boiling mixture at a concentration of 95.6% ethanol at 78.1 degC. However it appears that methanol does not form a constant-boiling mixture with water; see The Methanol Manual, on page 5. I'll leave it to someone who knows more physical chemistry than I do to evaluate what effect this would have had on the tests.
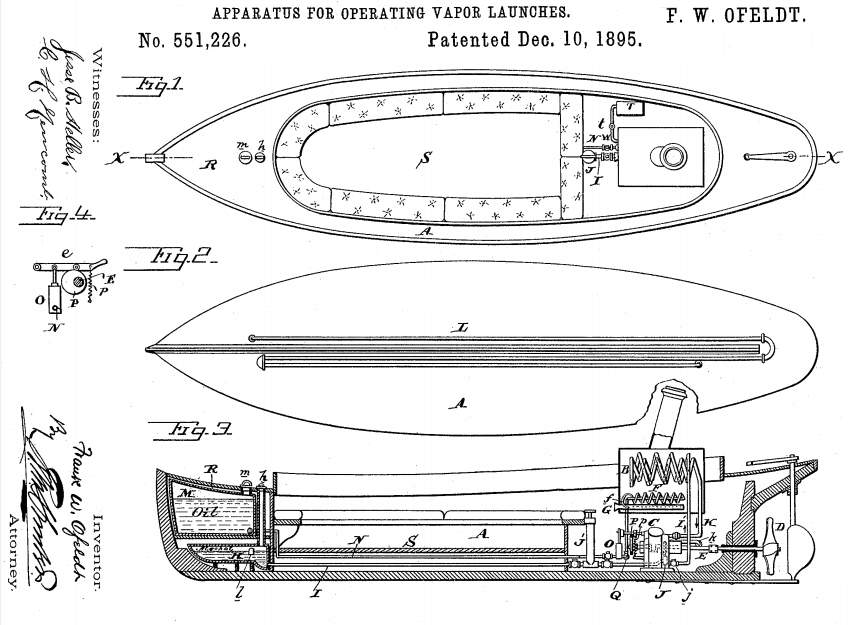
Above: Basic layout of an alco-vapor launch: from Ofeldt's US patent 551,226

Left: The front cover of the Marine Vapor Engine Company's catalogue



