Gallery opened: Jan 2007
Updated: 13 July 2017
More on the Malapert ether engine




Ether and Chloroform Engines |
Gallery opened: Jan 2007 |
[Webster's Dictionary, 1913]
Both ether and chloroform were tried as low-boiling-point working fluids in pursuit of greater efficiency. There are many different ethers, but the bare word "ether" usually refers to the well-known anaesthetic diethyl ether, otherwise known as ethoxyethane, CH3-CH2-O-CH2-CH3. (Not for the first time in this part of the museum, we are exploring the realms of organic chemistry) It used to be known as "sulphuric ether", because it was made by decomposing alcohol with sulphuric acid. It does not contain any sulphur atoms.
Ether engines are a very specialised taste in the field of power generation. Once again we have a working fluid which is both expensive and dangerous. Ether is well-known as an anaesthetic, but it is also extremely inflammable, having a low ignition temperature and being explosive over a wide range of concentrations; from 2% to 36%. The high density of the vapour means that it tends to accumulate dangerously in low areas. The boiling point of ether is only 34.6 °C, and so from a Carnot's Law standpoint, the efficiency of any engine using ether alone would be terrible.
If this is not discouraging enough, I have just discovered a whole new hazard in the use of ether as a working fluid. Diethyl ether oxidises and polymerises in the presence of air, creating the interesting compound diethyl ether peroxide (-CH(CH3)OO-)n. This is a colorless oily liquid that is an extremely brisant (fast-exploding) and friction-sensitive explosive; less than 5 milligrams can damage chemical apparatus. The dangerous properties of ether peroxides are the reason that the use of diethyl ether and other peroxide-forming ethers like tetrahydrofuran (THF) or ethylene glycol dimethyl ether (1,2-dimethoxyethane) is carefully avoided in industrial chemical processes.
I am certainly not an expert on ether chemistry, but continuously reboiling ether sounds like it might be a good way to generate the peroxide. Peroxides generally have higher boiling points than the compounds they come from, so if an ether mixture is heated, the peroxide can become progressively more concentrated and the risk of explosion increases rapidly.
Completely excluding oxygen from an ether cycle is not practical, especially since the ether condenser would presumably be at below atmospheric pressure, and so air would tend to leak inwards through glands, joints, etc.
The dangers of highly inflammable ether having been learnt the hard way, chloroform was also tried as a low-boiling working fluid, but without success. See bottom of this page.
Liquids that vapourise easily, when used in a stand-alone cycle, are not more efficient than water as a working fluid. Quite the reverse. See: Carnot's Law on the thermodynamics page.
EARLY BEGINNINGS
Sir Humphrey Davy suggested early in the 1800's that a volatile liquid could be boiled by exhaust steam and thus generate more power. This is called a "bottoming cycle".
The problem was studied by Ainger in 1830. So far I have found no detail of what he found out.
THE DU TREMBLAY ETHER ENGINE: 1850-56
Please note there seems to be no consensus on whether the inventor's name is spelled Du Tremblay or Du Trembley.
The first ether engine I have so far found was actually a combined water-ether steam engine, where the ether section was used as a bottoming cycle that extracted power from the heat rejected by the steam cycle. According to an article in Scientific American, (3rd September 1853) a Du Tremblay engine could be seen working at the Novelty Works (in New York?) in 1851. However, the journal Manufacturer and Builder, (in Jan 1875) citing the same Novelty Works as the location, said: "Practical difficulties of an insurmountable character caused the utter failure of the experiment." Unfortunately no details of what these difficulties were are given, and if they were talking about the same engine it seems strange that shortly afterwards it should have been installed in a vessel with some degree of success.
An ether engine was built in Marseilles to the design of M. Du Tremblay, under the direction of the noted French engineer François Bourdon. In 1850 the shipping line of Société Louis Arnaud, Touache Frères & Cie was founded, and they bravely chose to power their first vessel with Du Tremblay's combined steam-ether engine. It was even called the SS Du-Tremblay, indicating great confidence on the part of the owners. It was an iron schooner with an auxiliary screw driven by a steam engine of 70 nominal horse-power, though whether this figure includes the ether cycle is currently unconfirmed; presumably it did. The passenger capacity was 100 souls and she could carry 230 tons of cargo, so she must have been a sizable ship, though the actual dimensions are not yet to hand.
The engine had two cylinders, one for steam and one for ether. The steam cylinder exhausted into a multi-tube condenser with ether circulating in the tubes. This sort of condenser was itself unusual for the period as conventional marine engines used a jet condenser where the steam was condensed by a spray of sea-water; the low pressures prevailing at this time making it practical to use salt water in the boilers. The ether was presumably condensed by a multi-tube condenser cooled by sea-water.
The SS Du-Tremblay was held to be successful, with fuel consumption being said to be reduced by a third. Several other marine engines were then built during the years 1852-1855 and the innovation began to attract the attention of British engineers.
A correspondent, calling himself just "A SUBSCRIBER" wrote into Scientific American from Marseilles on the 5th of March 1856. (Before the France disaster described below) He states that the Du-Tremblay was of 65 horsepower, and there were two more boats actually working, the France and the Brazil, both of 300 horsepower. He says that more steam-ether ships were building; Zouave, Kabyle and Sahel, all of 200 horsepower, and Ville de Lyon and Amerique, both of 420 horsepower. Nothing more is known of these ships at present.
This chap seems to have been on the spot and had actual experience of the ether ships, for he says: "The waste of ether is almost zero- so little that the smell even is scarcely perceptible in the engine room." This does not appear to have been the experience of other users.
 | Left: The Scientific American report of the loss of the La France: 1856
On 27 September 1856, the France suffered an ether explosion and fire in the port of Bahia (on the coast of Brazil) after its first journey, and become a total loss; the company withdrew from transatlantic shipping. The incident put an abrupt end to the construction of steam-ether marine engines. Thanks to Peter Macinnis for drawing this article to my attention. |
The extract below is taken from a paper read by Sir Frederick Bramwell before the Mechanical section of the British Association, and published in the Scientific American Supplement, No. 312, December 24, 1881:
"Our president alluded to the employment of ether as a means of utilizing the heat which escaped into the condenser, and gave some account of what was done by M. Du Tremblay in this direction. It so happened that I had occasion to investigate the matter at the time of Du Tremblay's experiments; very little was effected here in England, one difficulty being the excise interference with the manufacture of ether. Chloroform was used here, and it was also suggested to employ bisulphide of carbon.
In France, however, a great deal was done. Four large vessels were fitted with the ether engines, and I went over to Marseilles to see them at work. I took diagrams from these engines, and there is no doubt that, by this system, the exhaust steam from the
steam cylinder, which was condensed by the application of ether to the surface of the steam condenser (producing a respectable vacuum of about 22 inches), gave an ether pressure of 15 lb on the square inch above atmosphere, and very economical results as regards fuel were obtained. The scheme was, however, abandoned from practical difficulties.
It need hardly be said that ether vapor is very difficult to deal with, and although ether is light, the vapor is extremely heavy, and if there is any leakage, it goes down into the
bilges by gravitation, and being mixed with air, unless due care is taken to prevent access to the flues, there would be a constant risk of a violent explosion. In fact, it was necessary to treat the engine room in the way in which a fiery colliery would be treated. The lighting, for instance, was by lamps external to the engine room, and shining through thick plate-glass. The hand lamps were Davy's. The ether engine was a bold experiment in applied science, and one that entitles Du Tremblay's name to be preserved, and to be mentioned as it was by our president."
The passage about "the excise interference with the manufacture of ether" is most intriguing. Had ether engines been practical, this would not be the only time that the British legal system got in the way of progess; the restrictions placed on early motor cars being the most shameful and notorious example.
But why were the excise men interested in ether? Presumably because it can be drunk to produce a state of intoxication. (I once knew a chap who derived great pleasure from sniffing ether, but that is perhaps taking us a bit too far off-topic) So far I have not really got to the bottom of this influence on ether engines.
THE SUSINI ETHER ENGINE: 1890-1893
This report appeared in several newspapers and journals:
"A Sulphuric Ether Motor"
"M. de Susini, a Corsican doctor, has, it is asserted, constructed a motive apparatus or propellor of 20 HP, which is worked by sulphuric ether, a result which the doctor anticipates will realise a saving of 65 per cent of the combustible material at present employed for setting machinery in motion."
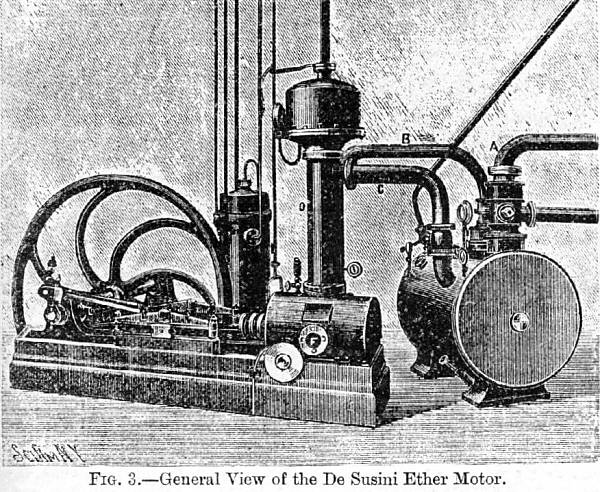 | Left: Susini's ether engine of 1893
|
The picture and the statement above are all the information currently known. Unfortunately it does not make complete sense. Why would two pipes (B and C) be required to carry the exhaust ether into the condenser? What is D? It is certainly not just a pipe, but looks more as if it might be some sort of level or pressure regulator. It has a pressure gauge attached at the top end. There is also the question of the circular drum to the right. The text implies it is the surface condenser, but I wonder if it might not really be the ether boiler, in which case A would be the steam inlet pipe and the thing D might be regulating the level of ether in the boiler. Note also the unmentioned pipe flange F on the side of the engine cylinder. This looks as if it might be the ether exhaust to the condenser.
The purpose of the five narrow pipes running vertically upwards is very uncertain. One of them looks as though it might be carrying ether vapour leaking from the piston-rod gland up to the roof and away on the breeze. The others come out of the mysterious dark cylinder to the left of D, and their purpose is equally mysterious.
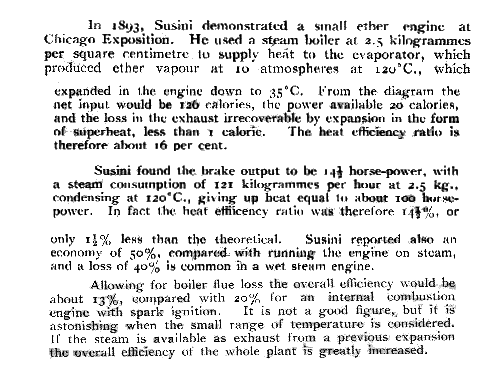 | Left: A report on Susini's ether engine of 1893
|
DESVIGNES DE MALAPERT AND ETHER: 1902
Susini may have been adventurous in using ether as a working fluid, but at least he didn't propose to heat it directly in a boiler. Monsieur Desvignes De Malapert not only proposed it, but appears to have done it. This exceedingly risky business was reported in English Mechanic & World of Science in January 1902, though the original publication was in France. The same issue also carried the picture of the Susini engine above. The full text of the article is given below, but in short there is no innovative answer to the clear and present danger of applying flame to an ether-filled boiler; De Malapert simply relied on tight joints for the boiler tubes. I for one do not find this very convincing.
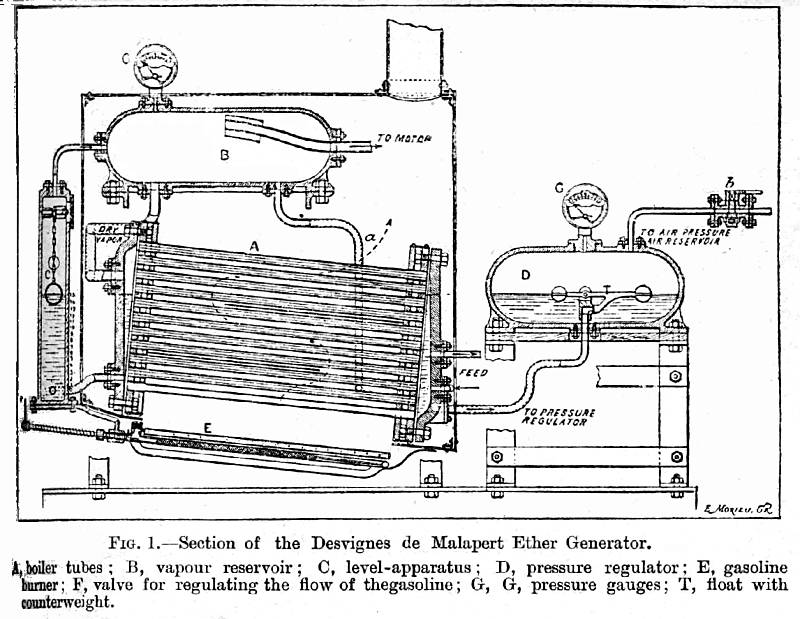 | Left: De Malapert's ether boiler
|
This seems a very doubtful system. It relies on the pressure in the boiler driving out all the liquid ether, so there is nothing left to boil and the pressure cannot rise further. When there is no water in a steam boiler the metal overheats, loses strength, and a boiler explosion results that is no less destructive than one caused by excess pressure. According to the English Mechanic article reproduced below, there was a feedback control loop from the pressure regulator to the fuel valve, so that should save the day; however safety is critically dependent on this piece of mechanism. If it fails the ether tubes will become red-hot when all the liquid is driven out, and I would expect them to fail under the pressure, releasing ether vapour into the furnace. I think we all know what comes next.
The fuel valve F is just visible at the lower extreme left, compressed by the effect of the book binding.
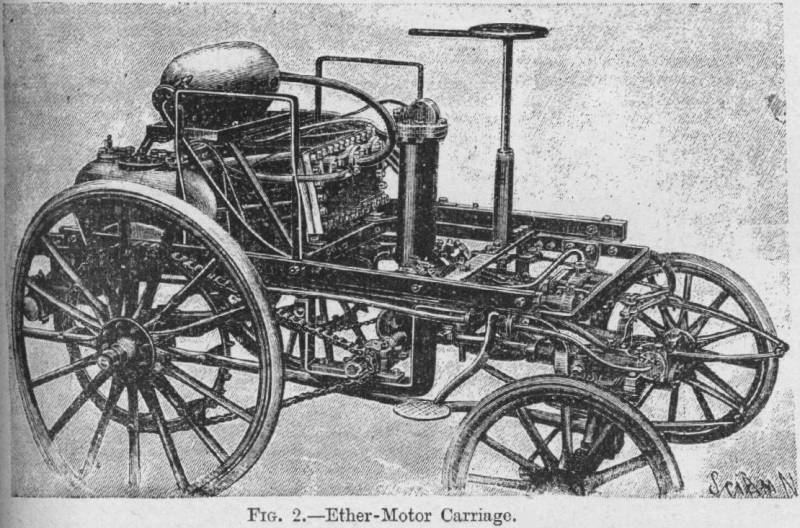 | Left: De Malapert's ether-powered car
|
De Malapert's name is unknown to Google. The dictionary definition of 'Malapert' is: adj: Saucy, quick with impudence.
You'd need to be impudent to juggle with boiling ether and naked flames.
Below is the full text of the 1902 English Mechanic article. You will note that the author seems to have no clue at all about Carnot's Law and the inevitable inefficiency of using low-boiling point working fluids, despite the fact that it was formulated as long ago as 1824.
 
| ||

| Apologies for the scanning difficulties. It is an old and fragile book.The article was originally published (in French, of course) in La Nature in 1901. It was also reprinted in Scientific American for 4 Jan 1902. | |
The De Malapert system was referred to in the book Modern Industrial Progress by Charles H Cochrane (pub Lippincott 1904), where it goes under the name of Desvignes:
"Ether has nuch theoretical advantage over steam for use in a motor, but the danger of fire has prevented its general use. Many experimenters have tried devices for making it sufficiently safe for commercial use. One of the most promising of these is the Desvignes ether generator. Since the boiling point of ether... is 97 degF, it follows that at a normal temperature of 67 degF only enough heat is required from the fuel to raise the ether by 30 degF as agaisnst 145 degF in the case of water, before the point is reached where power is obtainable. Of course more heat is necessary in either case to develop a pressure of vapor for use,but the calculation is that an ether motor wil use only about one-fifth the fuel required in a steam motor for giving out the same amount of energy."In employing ether the difficulty of the designer is too prevent too great heating of the ether, so as to create a dangerous pressure. In the Desvignes generator a feed-expansion regulator is employed, whose object is to limit the pressure in the generator. The vapor passes through a series of coils to the pressure-regulator. This contains compressed-air which has been provided at the pressure at which it is desired to run the apparatus. By means of a float-valve in the regulator, whenever the pressure in the generator rises above that of the compressed air, the generator discharges its surplus into the regulator, thus maintaining a balance of safety." |
This tells us nothing that is not given in more detail in the 1902 English Mechanic article, which was almost certainly its source. Charles H Cochrane also has no clue about Carnot's Law and the inevitable inefficiency of using low-boiling point fluids. Cochrane's main interest seems to have been in early attempts at aviation; several of the early pioneers such as George Graffigny thought that an ether engine would be useful, because it was presumed it would be lighter than a steam engine of the same power. This hope was illusory, being founded on the belief that ether engines were more efficient, when the reverse was true.
THE SHUMAN SOLAR ENGINE: 1906
Frank Shuman was an early pioneer of solar energy, like Henry Willsie. In 1897 he began experiments in which solar energy boiled ether in glass-topped boxes. In 1906 he produced a flat-plate collector design that was similar to Willsie's sulphur dioxide system except that it employed ether as a working fluid. One of these machines was set up pumping water outside his home in Tacony in 1907. The performance was poor; Shuman developed more efficient solar collectors that allowed him to abandon ether for steam, and eventually built a successful 50 hp solar power plant at Maedi near Cairo, Egypt in 1912, which pumped water for irrigation. Ultimately it proved not to be economically viable when imported coal became cheaper, and oil came into use. The outbreak of the First World War and the death of Schuman from a heart-attack in 1918 contributed to the abandonment of the project.
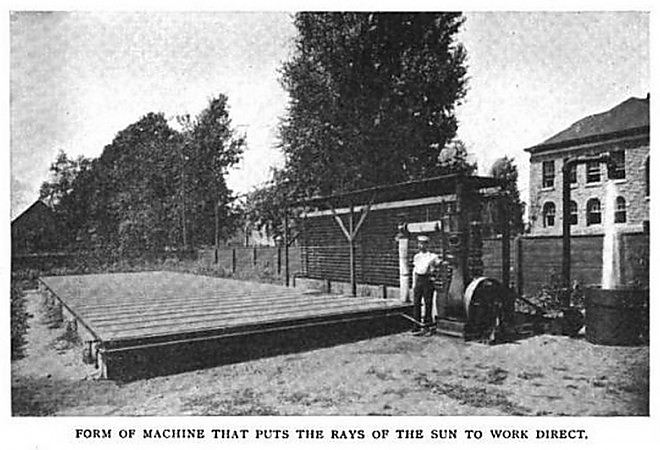 | Left: Shuman's Tacony installation: 1907
|
Shuman's steam engine sounds interesting in itself. The limited temperatures available meant that it had to work on low pressure steam, and Shuman claimed it would run economically on 4 psi absolute. Details of the solar boilers are given in US patent No 1 240 890 of 1917 (including the use of a thermopile to control mirror angle) but there is no information on the steam conditions. Clearly the power-plant would have been inefficent by conventional criteria, but there's little point in agonising about Carnot efficiency when the fuel is free.
Be aware that the Wikipedia entry for Frank Shuman shows little technical insight. Its author writes of "a full-scale steam engine that was powered by low-pressure water".
ETHER ENGINES TODAY
Isuzu Motors Limited seem to be planning to run an engine on di-methyl ether as a fuel: see US patent no. 6,742,479 (2004).
This is of course very different from using it as a recycled working fluid.

  
|