Gallery opened: 22 Aug 2012
Updated 24 Mar 2023
Stored-acetylene systems in Switzerland: 1941






Acetylene Engines |
Gallery opened: 22 Aug 2012 |
THE DANGERS OF ACETYLENE
If a substance will burn at all, you can bet that someone has tried to use it in an internal combustion engine. Acetylene is however one of the least promising substances as it has the disconcerting property of exploding with extreme violence if the absolute pressure of the gas exceeds about 29 psi. (200 kPa) Most regulators and pressure gauges read gauge pressure rather than absolute pressure so the safe limit for acetylene accepted today is actually only 15 psig (101 kPa). No other substance need be present for an explosion; oxygen is not required. The explosion is due to acetylene reacting with itself, typically forming benzene and/or vinylacetylene. These reactions are highly exothermic. Acetylene is however very useful for cutting and welding, and is therefore shipped and stored dissolved in acetone contained in a metal cylinder with a porous filling called Agamassan, which makes it reasonably safe to transport and use. However, it still has to be treated with considerable respect.
Acetylene can be liqufied easily, but in this state it is very dangerous. It is shock-sensitive and liable to explode violently. According to Edward Renouf in Popular Science, July 1899, a pressure of 39.75 atmospheres (584 psi) is required to liquefy acetylene at 20 degC, which is a bit out of line with its property of exploding at 15 psi. However there is no doubt that acetylene was liquified, apparently at the temperature of cold water, so this is at present a bit of a puzzle. In 1896 serious liquid acetylene explosions occurred at Pfeghar & Sons, New Haven, USA, and the factories of R Pictet in both Berlin and Paris. An explosion at the Isaac factory in Berlin caused several fatalities. Renouf was an enthusiast for liquid acetylene and flatly denied the indisputable formation of explosive copper acetylide, (see next paragraph) so perhaps his other information is equally suspect.
There is another, and more subtle danger. Copper piping was and is very widely used for plumbing of various sorts. If acetylene is contact with copper, it forms copper acetylide, which is a heat and shock-sensitive explosive when dry. When it goes off it naturally explodes the rest of the acetylene in the system, even if it has not been compressed. This is supposed to be the reason for the destruction of the lighting system at Lemburg railway station.
This sounded like an interesting incident, but it has so far proved hard to find anything out. Lemburg was the old Austrian name for what is now called Lviv in the Ukraine. The Polish name is Lwow. The current station opened in 1904, which sounds about the right era for an acetylene lighting system to be installed; at that time Lemburg was part of the Austro-Hungarian empire. In 1918 came the Battle of Lemberg between Ukrainian nationalists and Poles, ending very badly. In the course of this the railway station was captured by the Poles, no doubt suffering damage in the process, and by 1918 if the lighting system was being repaired electricity would probably have been used. Therefore, IF the explosion occurred in the current station, it must have been between 1904 and 1918. On the other hand, it may have occurred in the old neo-Gothic railway station built between 1861 and 1862, in which acetylene lighting could have been retrofitted.
When the danger was appreciated, following research by Grittner, (a man unknown to Google) many countries banned copper piping for acetylene, and also high-copper alloys. Hungary and Switzerland both banned alloys with more than 70% copper. Acetylene also forms explosive compounds with brass, (an alloy of zinc and copper) copper salts, mercury and mercury salts, silver and silver salts and nitric acid. Acetylene can come into contact with silver in silver-brazed pipe joints.
"Grittner’s gas was derived, at least partially, from a public acetylene supply..." is said on p101 of Acetylene, the Principles of Its Generation and Use (Leeds & Atkinson, 1910) which appears to show that at that time there were acetylene distribution networks, presumably for lighting. That was news to me. It may refer to public street-lighting rather than use in private homes. Notice also the reference to "a large village acetylene installation" in the Acetylene Motors: 1910 section of this page.
Compression of the fuel/air mixture before its ignition is essential for an internal combustion engine to have a reasonable efficiency. We can see that a fuel that explodes violently at only 15 psi gauge, even without air present, is not likely to be a good start.
Acetylene itself is not especially toxic but when generated from calcium carbide it can contain traces of highly toxic impurities such as phosphine and the even more horrible arsine.
If you're looking for something with almost as hot a flame as acetylene, (2925 degC rather than 3160 deg C) but much safer and easier to handle, you might consider MAPP gas.

The above difficulties did not deter a certain J K Rush, who wrote this rather vacuous article some time around 1905. He claims that acetylene was currently being used in engines:
"ACETYLENE GAS ENGINES""Until recently it has not been practical to use acetylene for gas engines, owing to the fact that but very few acetylene generators generate acetylene at a temperature low enough to obtain a purity of gas or quantity sufficient to bring about the practical use of acetylene in an engine, but there are some generators producing acetylene of a sufficiently low degree of temperature to bring about a purity of quality and increase of volume of acetylene to such an extent that cooking and heating with acetylene has not only been made practical and profitable to many who are now using acetylene, but its use is now applied very practically to engines, which have been formerly used with gas and gasoline." "Of course, engines used for this purpose are especially constructed, owing to the fact that a much smaller quantity of acetylene is required, when properly mixed with oxygen, to bring about good results in an engine than is used when coal gas is applied. A engine of this kind may be applied for running various kinds of machinery for factory purposes and the generator used for furnishing acetylene for heat, light and power. The heat may be used in the laboratory, the light for illuminating the entire premises, acetylene as applied to the engine, power for the entire institution - all supplied from one source. Te advent of the acetylene engine in the field of active industry will be a great boon to the trade generally, inasmuch as in many places acetylene generators will be purchased strictly for the sake of obtaining the gas for power purposes." "A country home or estate may now be fitted out with an acetylene plant, whereby the lighting of the buildings, as well as the grounds, is supplied from the machine, acetylene for heating and cooking purposes in the culinary department and hot water heating appliances in the bath room. The acetylene engine can be used for the purpose of forcing water through pipes in the most modern manner possible to conceive of, thus supplying the suburbanite with all the luxuries of city life so far as these particular items are concerned." "It is very interesting indeed to know the various uses to which acetylene is being applied. There is hardly a day at the present time but what some new application is made of this valuable combination of carbon and hydrogen. e see it in use on all up-to-date automobiles, launches, bicycles and many other similar uses, where the very brightest and best results are desired by way of illumination. ow, since the acetylene engine has come into the field, it would not be at all surprising to see within the next year at the automobile show, an automobile propelled as well as illuminated with acetylene." |
This article is taken from The Plumbers' Trade Journal reprinted in Amateur Work Magazine Volume 5, 1906".

ACETYLENE ENGINES IN GERMANY
The information in this section comes from ACETYLENE: A Handbook for the student and manufacturer by Vivian B Lewes FIC, etc. This was published in 1900 and was an important reference work in the acetylene business.
 | Left: Acetylene engine driving a water pump in Germany: 1900
|
 | Left: Table of engine fuel costs in Germany: 1900
|

ACETYLENE ENGINES IN FRANCE
 | Left: French advertisement for power from acetylene: 1900
|
 | Left: French advertisement for power from acetylene: 1900
|
 | Left: French advertisement for power from acetylene: 1900
|

THE ACETYLENE ENGINE OF CORNELIUS CRASTIN: 1896
 | Left: Cornelius Crastin and his acetylene-powered quadricycle: 1896
|
A little has been found out about Mr Crastin. His first name was Cornelius and he lived at 16 Tollington Rd, Holloway, London. He lived from 1852 to 1930. He wrote an article on acetylene and coal gas in Automotor Vol 03 (Oct 1898 to Sep 1899) p267. Crastin, C and Baldwin, G took out British patent No. 9928 (20 April 1897) for a 'Acetylene Generator for Lamps'.
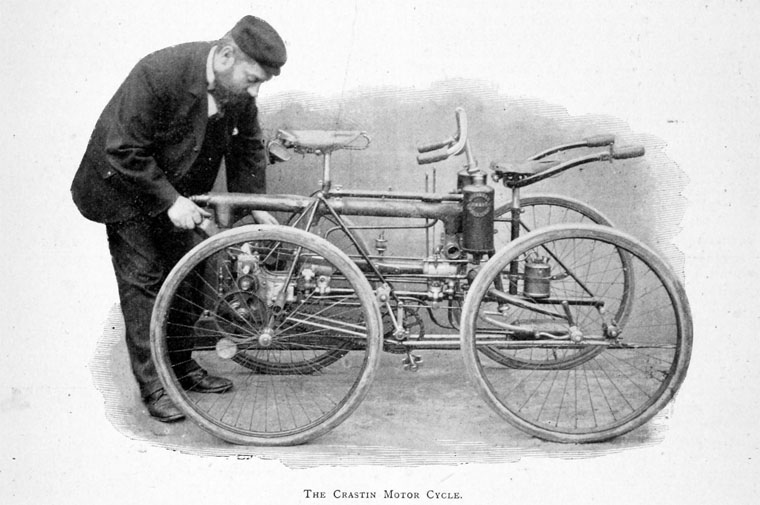 | Left: Cornelius Crastin and his acetylene-powered quadricycle: 1896
|

THE ACETYLENE ENGINE OF GUSTAVE WHITEHEAD: 1901
The aviation claims of Gustave Whitehead have always been controversial. He designed and built gliders, flying machines and engines from 1897 to 1915, but his claim to have flown a powered machine successfully several times in 1901 and 1902, (before the first Wright Brothers flights in 1903) are not generally believed.
However he unquestionably had a interest in engines of high power/weight ratio, Whitehead's Number 21 monoplane of 1901 had two engines: a ground engine of 10 hp (7.5 kW), intended to turn the front wheels and accelerate the aircraft to takeoff speed, and a 20 hp (15 kW) acetylene engine powering two contra-rotating propellers. Number 21 had a wingspan of 36 ft, (11 m) and fabric-covered wings were ribbed with bamboo, and braced by steel wires. The acetylene engine cannot have been very successful because Number 22 had a five-cylinder 40 hp kerosene engine.
Samuel Langley was the builder of the unsuccessful Aerodrome. His chief engineer, Charles Manly, was interested in Whitehead's Number 21 aircraft, on public display in Atlantic City, New Jersey in 1901. Manly said that what he was "more interested in than anything else" was the acetylene engine. Manly dismissed Whitehead's claims of flight.
Whitehead remains a controversial figure to this day, with a following of enthusiasts who believe he flew first. I have exploited this to gain a few more crumbs of information on his alleged acetylene engine. At gustave-whitehead.com they are concerned to show that Whitehead's acetylene engine was not an impossibility, and claim:
"In 1898, three years before Whitehead flew, Franz Liebertanz, General Secretary of the Calcium Carbide and Acetylene Gas Association in Düsseldorf, published a list of three known acetylene motors, one of them in America, in his 274-page book Calciumcarbid und Acetylen: ihr wesen, ihre Darstellung und Anwendung, pub Leipzig, 1898. The title means "Calcium carbide and acetylene: Their nature, their representation and application." This book is known to Google but there seems to be no way to view it without paying a ridiculous amount of money.
On 11th November 1899, Scientific American published a report about "The Auto Acetylene Company of New York". Its engines could be adapted for use with either acetylene, gasoline or kerosene:
"The motor consists of a duplex engine having four cylinders and two exploding chambers. There is a valve provided which permits changing from acetylene gas to gasoline and from gasoline to kerosene oil, so that while the engine is operated most economically and satisfactorily with acetylene, at the same time other fuels can be used in an emergency, if supplies of carbide are not readily obtained."
Technical drawings of an acetylene motor in a road vehicle were published in 1899 in Dingler's Polytechnisches Journal, Vol. 7, pp. 111-112.
 | Left: From an American newspaper: 1900s
|
 | Left: News item from the New York Herald: 1901
|

AN ACETYLENE ENGINE FOR A HELICOPTER: 1902
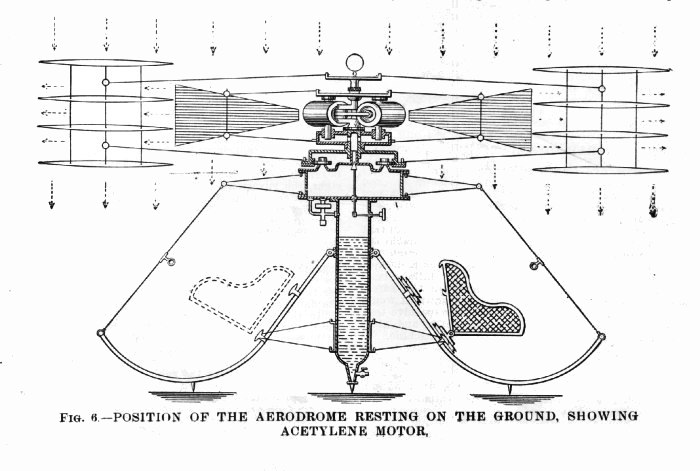 | Left: Proposed helicopter with acetylene engine: 1902
|
The helicopter is, as far as I can see, at best a death-trap, with nothing to stop the passenger section from going round in the opposite direction to the rotors. But what we are concerned with here is the power plant: "...a form of prime-mover is shown using acetylene or other suitable gas compressed or liquefied. Or the liquid gas may be absorbed in acetone as is now being introduced for the lighting of railroad cars. The retainer is a tube, shown seven inches in diameter, to contain 1,386 cubic inches of the liquid, which on expanding to atmospheric pressure, will make over 321 cubic feet of gas which will furnish thirty horsepower for about two and a half hours. Authority for this statement is the data contained in the works on gases by Lewes, Frank, and Hiscock.* The pressure in the liquid container is reduced in the intermediate chamber from 27 to 12 psi by a regulator."
That's about all that is relevant to the engine.
From the drawings the engine is a multi-cylinder rotary type, ie it goes round with the rotor. Tedious details like the ignition system are omitted. Later in the article there are references to the engine "...using steam, carbon dioxide, liquid air, or other expansive non-explosive gas...". Truly a multi-fuel engine. Clearly Mr Mott was not fixated on acetylene as his fuel.
Looking for Mr Mott was an interesting business; at first only a few faint traces were found. He appears as witness to a patent by J.D. Galloway in 1886, and he himself took out US patent 444,454 for a Racing Indicator. However gold was struck in the shape of an article in the New York Times headlined "Passaic Inventor Thinks He Has Practical Flying Machine" which is pretty much a rehash of the Scientific American article, and tells us no more about the acetylene engine. Nothing has so far been built and Mr Mott is looking for funds...
Later the irrespressible Mr Mott came up with US patent 1,330,274: "Combined Helmet And Parachute For Aviators' Use" in 1918. It looks about as practical as it sounds.

* Vivian Byam Lewes (1852-1915) was the author of Acetylene; a handbook for the student and manufacturer published in 1900. He was Professor of Chemistry at the Royal Naval College, Greenwich. This book can be easily found and downloaded; there is some data on getting power rather than light from acetylene, stating at p601, which begins: "There are several investigators at present working onthe utilisation of acetylene for gas motors, but there are many difficulties to be overcome before this is successfully accomplished, as the deposition of carbon when the air supply is insufficient, and the violence of the explosion, are troublesome factors to deal with."
Frank seems to be Adolph_Frank co-inventor of the The Frank–Caro process, also called the cyanamide process, the first commercial process for nitrogen fixation.
Hiscock has so far eluded the Museum staff.

THE BARKER-WHITE ALCOHOL-ACETYLENE SYSTEM: 1907
The Barker-White system ran an engine on a mixture of alcohol and acetylene. An ordinary carburettor gave an alcohol/air mixture, and the system then relied on the water that made up about 10% of the current commercial alcohol, to react with calcium carbide and generate acetylene. This made the alcohol burn more rapidly while the alcohol suppressed the exposive tendencies of the acetylene. Relying on what must have been a variable amount of water in the alcohol to set the critical amount of acetylene burnt sounds very dodgy to me.
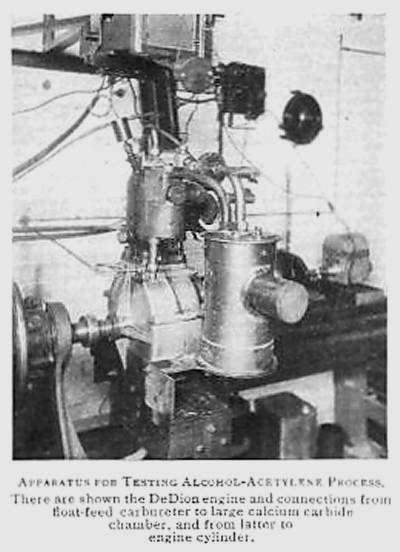 | Left: Barker-White test engine: 1907
Source currently unknown |
 | Left: Barker-White engine test: 1907
Source currently unknown |
ALCOHOL-ACETYLENE GAS FOR MOTOR VEHICLES
Report on the Barker-White System from "Motor Age" New York, 11th April 1907. It also appeared in "Commercial Motor", apparently on the same date.
"All attempts to use denatured alcohol as fuel for the motor in test runs made with motorcars have clearly demonstrated the unsuitability of the new hydrocarbon for use in the high-speed, low-compression motorcar engines now manufactured. It is impossible to start cold on alcohol alone, because it is much less volatile than petroleum spirit and has a higher ignition temperature, so that without the application of external heat the air taken in through the carburetter will not evaporate enough alcohol to form an inflammable vapour. When the engine has been started on petroleum spirit and warmed up to a temperature at which alcohol would vaporise satisfactorily, it was determined that the compression of 60 to 70 pounds per square inch in the engine was insufficient for the best results, and that the alcohol vapour burned so slowly that much of the energy was lost in the exhaust when running the engine at normal speed.""Stationary alcohol engines, which have been brought to a high state of perfection in France and Germany, have compression as high as 120 pounds, and operate most efficiently at from 200 to 300 revolutions per minute. As a result of these conditions, there was a great waste of fuel in the motorcar test runs and, although they showed that the cars would run on alcohol alone after being started, they failed to demonstrate any economy in the use of alcohol, even upon the assumption that eventually the new fuel will be marketed at a price to compete with petroleum spirit, as the cars would not run more than seven miles on a gallon. To overcome these objections, and to make it possible to utilise commercial alcohol in the present type of internal-combustion engine, without any alteration further than a change of carburetters, or the addition of a supplemental device, a new process known as the Barker-White system was invented and patented last year by F W Barker and Thomas L White, of New York City." "During the past winter, a series of laboratory experiments with this system has been made by Joseph Tracy, until results have been attained that warrant the statement that the new fuel develops as great efficiency in a given engine as petroleum spirit, and that the motor can be started cold with it. The invention is, broadly, the process of producing a gas for power purposes which consists in carburetting air with dilute alcohol and then bringing such carburetted air into contact with calcium carbide. By passing the vapour of alcohol and air as formed in the ordinary spray carburetter through a layer of calcium carbide, a variety of effects, all desirable, are secured. The first is to extract from the alcohol the greater part of the water contained in it, which, in commercial alcohol, is present to the amount of in per cent or more. As water has no fuel value, and its only effect when present in the cylinder is to reduce the temperature and, consequently, the efficiency of the engine, it is altogether desirable to remove it. This is done by the carbide, which dehydrates the vapour on its way to the cylinder. As the water unites with the carbide, it is dissociated into hydrogen and oxygen, the former combining with the carbon in the carbide to form acetylene gas, and the oxygen uniting with the calcium chloride and forming calcium oxide or lime. The chemical reaction generates heat, which assists further to volatilise the alcohol before it enters the explosion chamber, and the liberated acetylene gas commingles with the alcohol vapour, effecting both a mechanical mixture and a subtle chemical combination, the exact nature of which has not yet been determined. Acetylene burns with great rapidity, offsetting the slow burning of the alcohol, the resulting effect being that the whole charge burns much more quickly than would alcohol vapour alone. In brief, the two gases have a counteracting effect that can, by proper regulation of the quantity of water present in the alcohol, be made to approach very closely the properties of the ordinary petroleum spirit. The more nearly one can approach to complete combustion at the instant of maximum pressure, or minimum volume, utilising the entire downward stroke of the piston for expansion only, the greater the efficiency developed." With the alcohol-acetylene mixture, which has been given the distinctive name " Alkoethine," combustion occurs early in the piston-stroke, and only expansion later, so that, before the exhaust valve opens, combustion has been completed, which is not true of alcohol vapour when the engine is run at high speed." "A decided advantage is that an excess of water in the alcohol as purchased is not an objection; on the contrary, the greater the quantity of water the more rapidly the engine will run. In the tests as carried out by Mr. Tracy, with the single-cylinder De Dion engine of 3 to 31 horse-power, it has been found that the addition of water to up to a total of 18 per cent. gives the best results, increasing the proportion of acetylene gas in the ultimate explosive mixture to an amount that gives results most nearly approaching these of petroleum spirit. There is, however, a wide latitude of choice, ranging from alcohol undiluted to a solution containing 25 per cent. of water. Since the addition of water increases the bulk of the fuel, and water costs nothing, it offsets the cost of the carbide consumed." "No quantitative tests have yet been made with the apparatus, which is merely a large brass cylinder Connected with the cylinder of the engine, and with an ordinary float-feed spray carburetter, by copper piping, and has a 'diaphragm of wire netting upon which lies the layer of calcium carbide of quarter-inch size. Therefore, the proportions of carbide and water used to a gallon of commercial alcohol consumed to produce a given indicated horse-power have not been determined; roughly, however, about 1 pound of carbide is used to one gallon of the IS per cent. solution of alcohol and water. In the experiments that have been conducted it has been found that all the heat that could be collected from the exhaust by means of a long sleeve is just sufficient fully to vaporise the alcohol in the carburetter. To start cold the engine is " primed " by throwing a wine-glass full of alcohol solution directly into the carbide chamber to generate an excess of acetylene. The chemical action liberates heat from the carbide, which further evaporates the alcohol just before it enters the engine cylinder. As pound of carbide contains goo British thermal units, and there are approximately 450 British thermal units. of latent heat in I pound of alcohol, the decomposition of a pound of the former will supply enough heat to evaporate fully 2 pounds of alcohol from a liquid state. The size of the carbide lumps and their disposition in the carbide chamber are of considerable importance, as the vapour must pass through the mass freely, and, at the same time, come in contact with a large surface of the carbide. Experiments are to be made in the near future to ascertain what size will give the best general results, and to settle a few other important constructional details." "It has been found that perfect combustion of the mixture takes place through a wider range of proportions of gas and air than is the case with petroleum spirit. With petroleum spirit and air the range is from one part of liquid to eight parts of air to one part of liquid to fourteen parts of air, whilst with pure acetylene and air the range is from one to three all the way to one to twenty-two parts. The range with the alcohol-acetylene combination is about halfway between these, varying according to the quantity of water contained in the alcohol. "Alkoethine" can be regulated so that it will ignite at the same sparking point as petroleum spirit, whilst with pure alcohol, which is slow burning, it is necessary to advance the spark much more. When the mixture begins to get so rich that it deposits soot, ignition becomes difficult and even ceases at times." "Indicator diagrams taken with manograph instruments during the tests, and at the demonstration before the Press representatives on March 5th, showed results quite as good as cards taken from the same engine using petroleum spirit. With a compression of 65 pounds, and advanced ignition, a very high peak was obtained; with maximum pressures. of more than 240 pounds. Whilst the engine used developed highest efficiency at 1,100 revolutions per minute when running on petroleum spirit, with " Alkoethine " the efficiency. did not decrease until i,eoo revolutions per minute were attained, and, whilst the maximum speed on petroleum spirit was 1,700, the motor has been run up to 2,100 on the alcohol-acetylene combination." |
This report is interesting, not only because it introduces us to "Alkoethine" but because it makes clear that the acetylene was used to make alcohol an acceptable fuel, and not the other way around.
ALCOHOL-ACETYLENE GAS FOR MOTOR VEHICLES: Second report.
From "Commercial Motor" 2nd May 1907
"In our issue of the 38th ultimo, page 384, we referred to some very interesting experiments which had been made in the United States of America by Messrs F W Barker and L White, of New York, during which research some instructive facts were brought to light. |
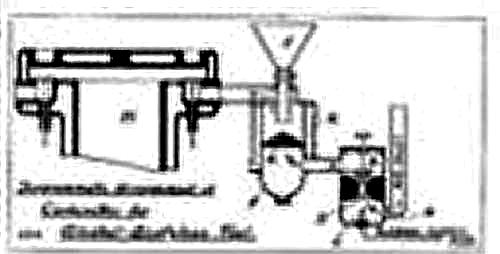 | Left: The Barker-White fuel arrangements: 1907
|
ACETYLENE FOR MOTIVE POWER: 1909
This brief report was published in the Bulletin of the Department of Chemistry of the The Pennsylvania State College in 1909, by George Gilbert Pond, PhD, Professor of Chemistry
"An interesting field for a display of inventive genius is the application of acetylene for the generation of motive power. The high temperature of its combustion, affording a much greater explosive power than coal gas or gasoline, and the trifling trouble and expense involved in generating and storing this fuel should work together to render its use in gas engines of great benefit to the mechanical engineer. Suitably contrived engines or motors are a prime requisite and such appliances must come, but for some reason really brilliant progress along this line has been deferred. This is in spite of the fact that the efficiency of the acetylene explosion, as compared with coal gas, is about as three to one. Acetylene will eventually demonstrate its superiority in this line, perhaps is already doing so, but the difficulties seem to be slower of remedy than in the other uses to which acetylene is applied. The blowpipe was not prompt in making its appearance, but the difficulties were finally overcome, and so they will be, must be, in case of the successful acetylene motor of the future.""One interesting development in this direction is the alcohol-acetylene motor for vehicles. This invention is, in broad terms, "the process of producing a gas for power purposes which consists in carburetting air with dilute alcohol and then bringing such carburetted air into contact with calcium carbide." Such dilution of the alcohol is employed as may be determined by experiment to be most advantageous, and when this is sprayed through a layer of carbide, the water is extracted from the dilute alcohol, rendering the latter more efficient as a fuel, and converted into acetylene by reaction with the carbide. The dry gaseous mixture of alcohol vapor and acetylene passes to the explosion chamber and burns far more advantageously than alcohol vapor alone. The qualities of the two substances supplement each other, and the mixture is a fuel of considerable efficiency; it is probable that the inventive genius which is now being applied to this problem will in due time develope its full efficiency." |
This is very probably another reference to the acetylene-alcohol mixtures of Barker and White, as described just above.
ACETYLENE MOTORS: 1910
 | Left: Extract from From Acetylene, the Principles of Its Generation and Use by Leeds & Atkinson, 1910
|
Leeds and Atkinson also give us a report on tests with carburetted acetylene:
"Lepinay has described some experiments on the comparative technical value of ordinary acetylene, carburetted acetylene, denatured alcohol and petroleum spirit as fuels for small explosion engines. One particular motor of 3 (French) h.p. consumed 1150 grammes of petroleum spirit per hour at full load; but when it was supplied with carburetted acetylene its consumption fell to 150 litres of acetylene and 700 grammes of spirit (specific gravity 0.680). A 1-1/4 h.p. engine running light required 48 grammes of 90% alcohol per horse-power-hour and 66 litres of acetylene; at full load it took 220 grammes of alcohol and 110 litres of acetylene.A 6 h.p. engine at full load required 62 litres of acetylene carburetted with 197 grammes of petroleum spirit per horse-power-hour (uncorrected); while a similar motor fed with low-grade Taylor fuel-gas took 1260 litres per horse-power-hour, but on an average developed the same amount of power from 73 litres when 10 per cent. of acetylene was added to the gas. Lepinay found that with pure acetylene ignition of the charge was apt to be premature; and that while the consumption of carburetted acetylene in small motors still materially exceeded the theoretical, further economics could be attained, which, coupled with the smooth and regular running of an engine fed with the carburetted gas, made carburetted acetylene distinctly the better power-gas of the two." |
From Acetylene, the Principles of Its Generation and Use by Leeds and Atkinson, pub Griffin 1910, 2nd edn p263
This book can be downloaded for free from the Cornell Library.
Acetylene was 'carburetted' by saturating it with petrol vapour in a crude carburettor. Possibly this retarded its tendency to explode on compression. It is not surprising that Lepinay found that "with pure acetylene ignition of the charge was apt to be premature." What is perhaps surprising is that the whole engine did not explode.
Despite using the full resources of the Interwebs, all that has been discovered so far about M'sieur Lepinay is that his first initial was "A".
THE SWISS ACETYLENE ENGINES: 1942
Several Swiss companies began developing systems for running cars on acetylene in 1939, as it became clear that even if you were a neutral country, petrol was going to be in very short supply.
Here is an extract from an article in the Canberra Times for 7 April 1942:
"ACETYLENE AS A MOTORFUEL" |
Felix Brun pointed out to me an article in Schweizerische Bauzeitung, for 3rd May 1941, on the use of acetylene in Switzerland. (text in German) It can be seen at retro.seals. The article describes several wet and dry acetylene systems available in Switzerland at the time. I assume that a "wet" system is one that generates acetylene on-board by dropping water on carbide, while a "dry" system carries acetylene stored in cylinders.
It is noted that the systems were quite expensive to run, and the gasification of the coal is a cheaper way to run a car without gasoline. (Assuming of course you can get hold of the coal) In any case, coke for carbide production still had to be imported and that may have been why acetylene was banned later in the war. The author says that most of the car installations were poorly built and dangerous, and the federal tester had to reject most of those presented to him.
Felix very kindly translated the article summary on Page 206 for me:
"Acetylene for operating a vehicle is produced by the reaction of water on calcium carbide, and while on the car itself in peculiar generators or fixed installations, and then compressed in bottles and in acetone dissolved to be carried.According to its characteristics acetylene as a replacement fuel for automobiles is far from ideal. Modern automobile engines can when operating with pure acetylene only be regulated to about half of the power with gasoline, because otherwise at spontaneous combustion in the engine occurs at approx 365°C with corresponding palpitations occur. The addition of liquid with a high heat of vaporization increases the knock limit and allows benefits from 75 to 85% of the gasoline power extracted. As damping materials are particularly suitable water and methyl alcohol, and mixtures thereof. They cool the gas mixture and the entire compression chamber. Nevertheless are essential special spark plug with high heat value.
Preventive measures. Acetylene is not poisonous only in high concentrations (low oxygen) it is anesthetic." |
Note that a safety valve working at 1.5 bar (22psi) is mandatory. Presumably that must be absolute rather than gauge pressure, so it would vent at about 7psi gauge giving a safety margin of 8 psi over the maximum pressure of 15 psi gauge. Also, the Upper Explosive limit (UEL) with air is given as 65% rather than the modern figure of 81%.
There is a mine of information here.

ACETYLENE GENERATORS
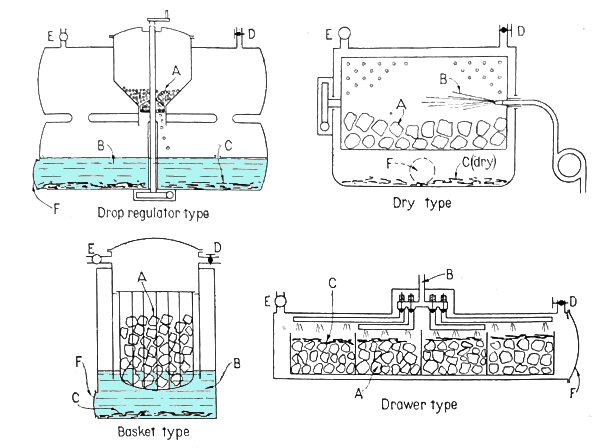 | Left: Acetylene generator systems: 1941
|

There now follow some illustrations of acetylene installations in vehicles. The manufacturers involved are presumably those mentioned above as being at "a recent motor show in Zurich".
THE ERIS ACETYLENE SYSTEM: 1941

 | Left: The Eris acetylene system: 1941
|
THE EXCELSIOR ACETYLENE SYSTEM: 1941

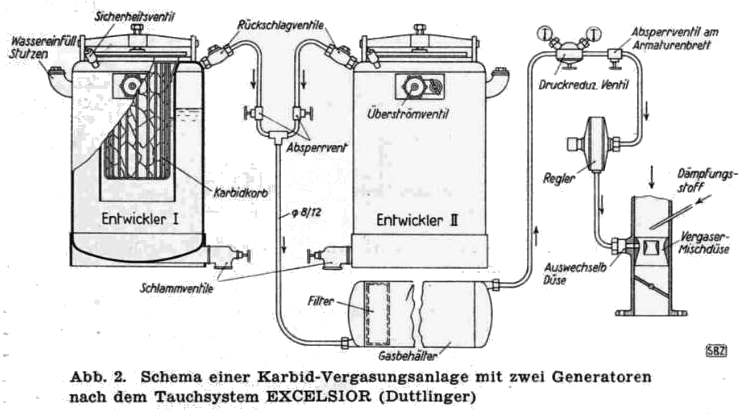 | Left: The Excelsior acetylene system: 1941
|
Entwickler means "developer" or generator.
Absperrvent means "shutoff-valve".
Schlammventile means "mud-valves".
Druckreduzventil means "pressure-reducing valve".
Quite what the advantage of having two generators was is currently unclear; you were hardly likely to keep driving on one generator while the other was being recharged with fresh carbide. See also the RIWA system below, which also had two generators. In the Excelsior diagram above each generator has its own safety-valve (sichercheitsventil) venting to atmosphere.
 | Left: Excelsior acetylene system: 1941
|

THE RIWA ACETYLENE SYSTEM: 1941
 | Left: RIWA acetylene system: 1941
|

THE ENDRESS ACETYLENE SYSTEM: 1941
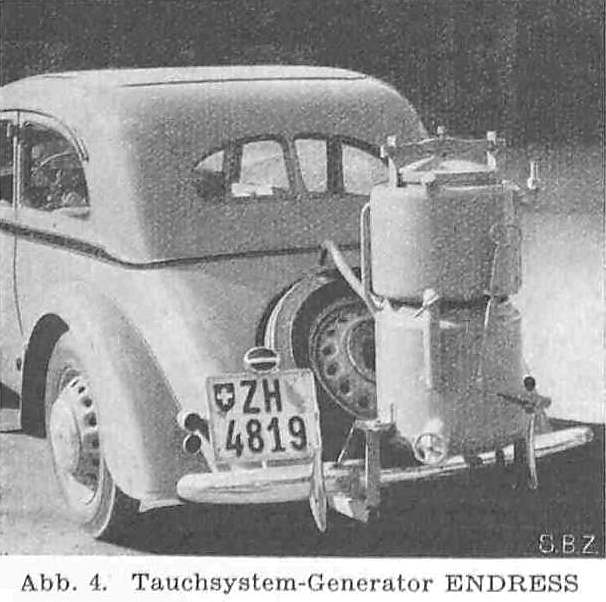 | Left: ENDRESS acetylene system: 1941
|

THE BUSS ACETYLENE SYSTEM: 1941
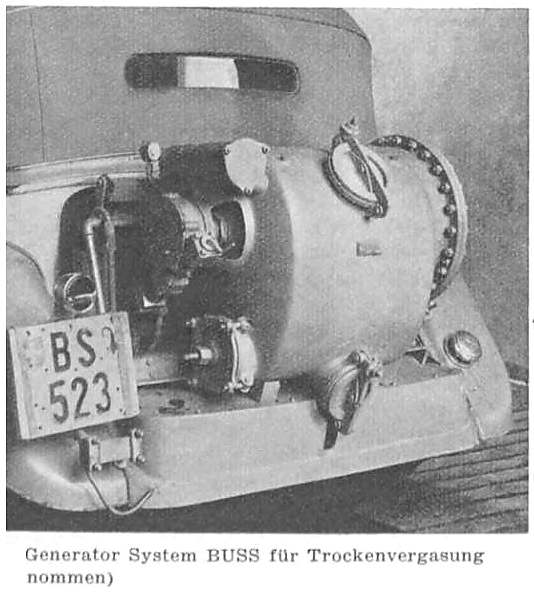 | Left: BUSS acetylene system: 1941
|
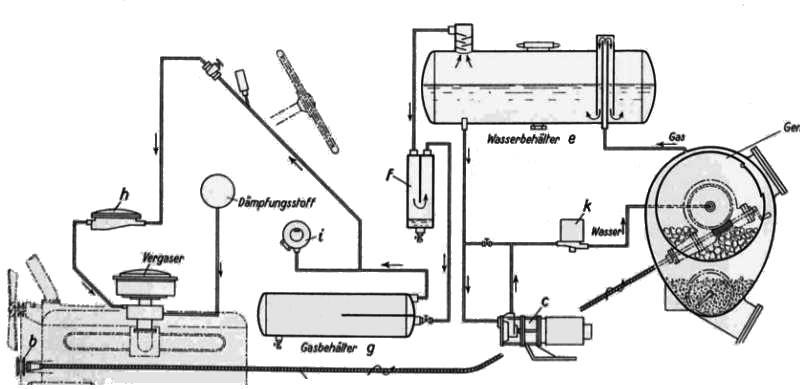 | Left: BUSS acetylene system: 1941
|
Note the wormwheel and handle for rotating the inner carbide drum; this seems to have a hatch that would have to be lined up with the filler cap so more carbide could be added. It appears the inner drum needed to be rotated when you were motoring along, as it is driven by what appears to be a flexible cable connected to pulley b on the engine at the bottom left.
Just before the carburettor ("vergaser") is the pressure regulator h. Item i is rather mysterious; in the article it is labelled as "regulation of the water inflow through the pressure regulator" according to Google Translate. J Kreutz suggests it is a pressure switch controlling the valve "k" and/or the pump "c", and this seems very probable.
The two unlabelled items just in front of the steering wheel appear to be a pressure gauge and a shut-off valve. There is also a circle labelled "dampfungsstoff" which defeats Google but I think roughly translates as "steam stuff". I imagine it is a reservoir of water (or possibly an alcohol/water mixture) which was injected into the acetylene to prevent detonation.
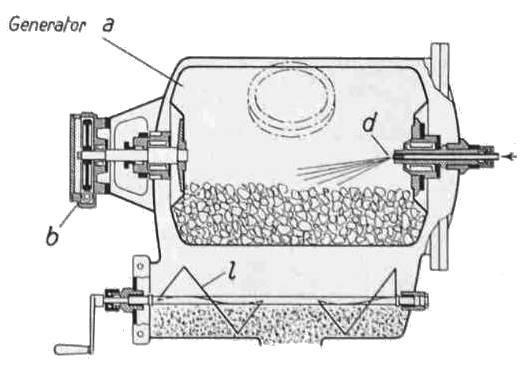 | Left: BUSS acetylene system: 1941
|
The Buss company was founded in 1884 by Albert Buss as a structural steel company, and began its chemical engineering activities in 1919.
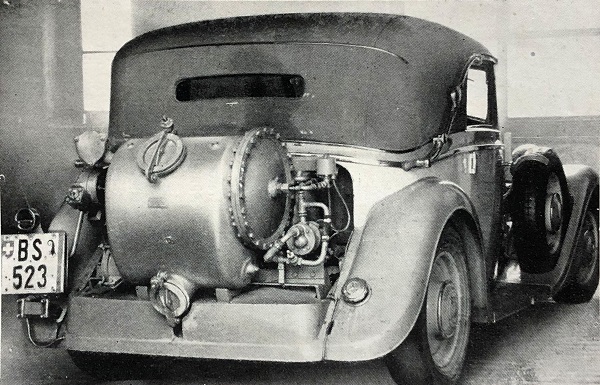 | Left: BUSS acetylene system: 1941
|

THE CARBURO ACETYLENE SYSTEM: 1941
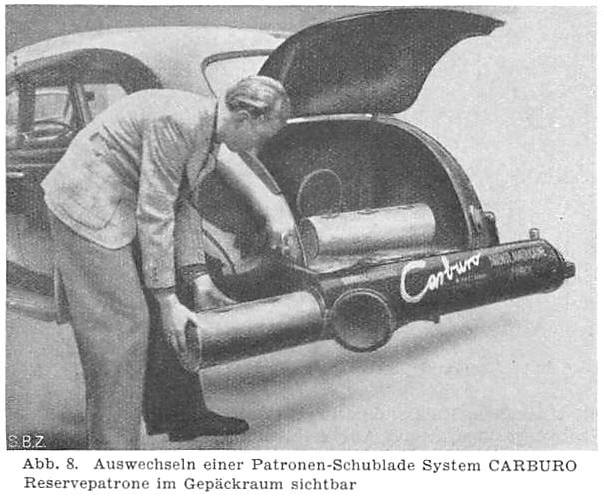 | Left: The Carburo acetylene system: 1941
|
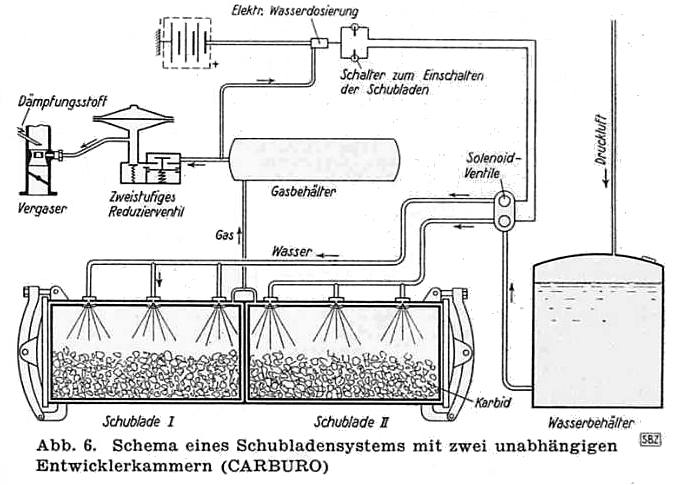 | Left: The Carburo acetylene system: 1941
|

THE GENERAL MOTORS CARBOR ACETYLENE SYSTEM: 1941
This satisfyingly complicated diagram of an acetylene engine was published in Newnes Practical Mechanics for February 1942, in the middle of the Second World War, as part of an article titled "The Properties of Acetylene-Gas Fuel". The accompanying text however only refers occasionally to the diagram and almost all of it consisted of a recitation of the well-known properties of acetylene, which could have been copied from any 30-year-old textbook. (such as that by Leeds and Atkinson, mentioned just above) Combining that with the fact that technical magazines like Practical Mechanics would had to have been very cautious about what they published in war-time, I began to suspect that the article might have been deliberately designed to mislead the enemy, and induce him to put resources into investigating an explosively impractical idea. But read on...
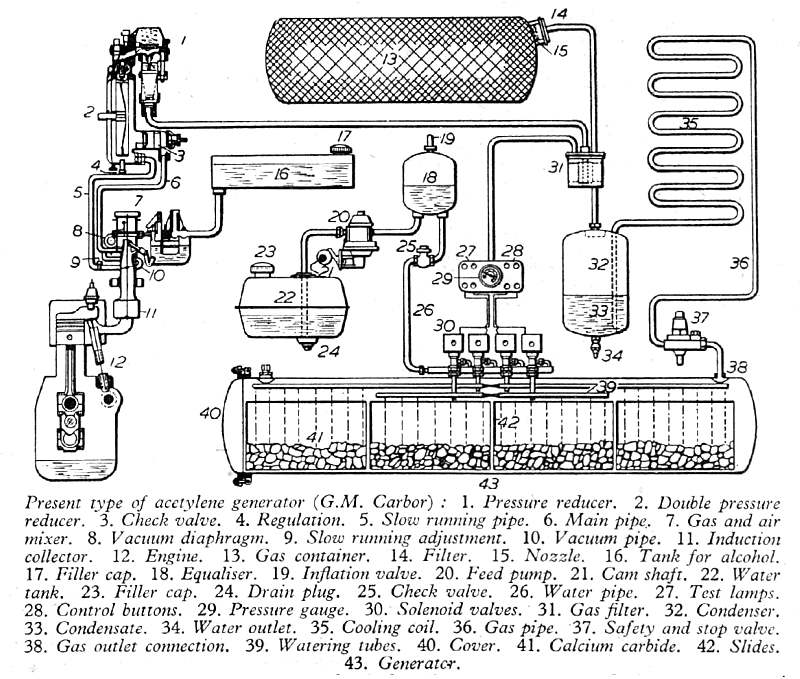 | Left: Swiss acetylene-fuelled engine system: 1942. Note the source of the diagram is "G. M. Carbor".
|
The acetylene is generated in tank 40, by the usual method of dropping water on calcium carbide; the tank is divided into four compartments, and water supply to each is controlled by solenoid-operated valves controlled from the dashboard. 22 is the water tank. 13 is a reservoir for the acetylene as gas generation can only be started and stopped slowly; the acetylene is dissolved in acetone absorbed in charcoal. The acetylene goes through pressure-reduction valves 1, 2 and into the gas/air mixer 7, which is also fed with alcohol from tank 16. The mixture is then used in engine 12, presumably with some alterations in ignition timing.
Steam is generated when acetylene is produced from carbide, and this was condensed in 35, then returned to the water system via vessel 32.
The actual fuel was therefore an acetylene-alcohol mixture, and the text states that without the alcohol the acetylene-air mixture was very weak, (to avoid detonation) and so gave little power. The acetylene/alcohol ratio is not given, but one suspects that a lot of alcohol was needed. It was also mentioned that 3 parts of alcohol to 1 part of water could be used instead of pure alcohol. Note that an acetylene/alcohol mixture was used by Barker & White in 1907, as described above.
Part 37 in the diagram above is a "safety and stop valve" which presumably vented to atmosphere if the pressure became excessive. (The "stop" function presumably means it stops pressure build-up after the engine has stopped) As noted, dropping water onto carbide is not a process that can be stopped instantly. If the reservoir tank 13 became saturated, it would be vital to relieve the pressure before it reached 15 psig and the whole system exploded mightily. This safety valve might not be that safe, as you would be venting an extremely flammable and explosive gas. The Lower Explosive Limit (LEL) of acetylene is a concentration of only 2.5% in air while the Upper Explosive limit (UEL) is 81%, a wider range than almost any other gas, (Exceptions are exotic things like diborane: 0.8 % to 88%) and this greatly increases the chance of something bad happening if acetylene is vented near a running internal-combustion engine.
 | Left: Acetylene-powered motorcycle: 1941?
|
STORED ACETYLENE SYSTEMS: 1941

 | Left: Stored-Acetylene car: 1941?
|
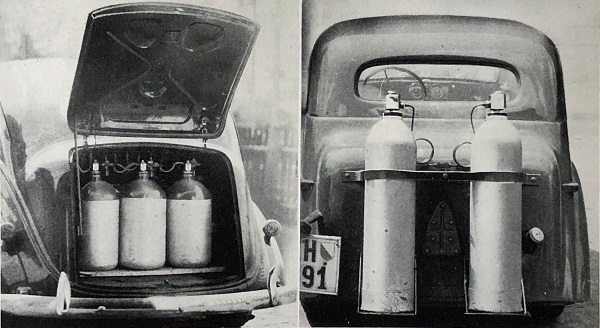 | Left: Stored-Acetylene cars: 1941
|
ACETYLENE-POWERED PANZERS: 1944
By 1944 Germany was experiencing crippling shortages of fuel for motor vehicles. In that year Panther tanks we fitted with acetylene bottles for use in training at Eisenbach in Thuringia, the base for the 1st Panzer Division. Presumably some modification of the engine was required; normally its Maybach engine ran on petrol. Turning coal into acetylene is much easier than turning coal into petrol.

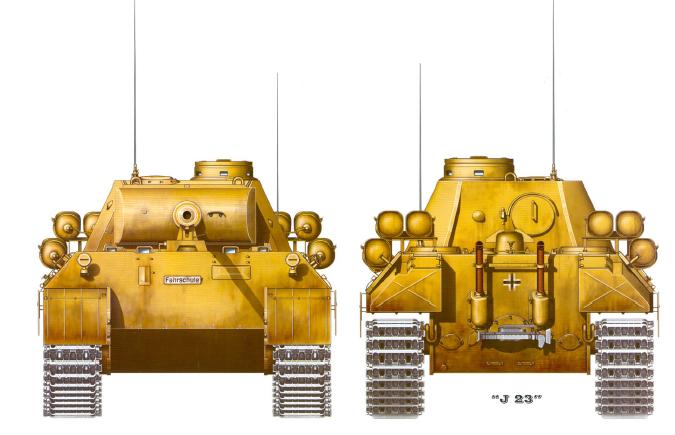 | Left: Acetylene-powered Panther tank: 1944
|
Diligent research by the Museum staff has been able to uncover little more than the bare existence of the drawings shown here. They were found by Google but is possible that they come from AJ Press Panther Vol 3 by Andrew Dextras, and if so I must apologise in advance. I suggest that this vehicle was a one-off, and that the cylinders actually contained natural gas; that would at least explain their horizontal installation. On the other hand, note the horizontal acetylene reservoir used by the Swiss system above.
 | Left: Acetylene-powered Panther tank: 1944
|
ACETYLENE AS A WEAPON AGAINST ENGINES
In various odd corners of the internet, there are statements that acetylene can be used as a weapon against military vehicles. Here is a sample:
"By exposing a diesel engine to a concentration of just 3% acetylene, such severe pre-ignition difficulties result that the engine self-destructs in a matter of seconds. Without mobility, even the most sophisticated muli-million dollar tank is just a heap of scrap metal. This system has already been designed and tested, formed from a binary chemical warhead composed from calcium carbide and a water gel."
I have so far been unable to confirm or deny whether this is real or total fantasy. For the time being, I give it to you for what it is worth. As noted above, a concentration of 3% acetylene is explosive.
PRACTICALITIES OF ACETYLENE POWER
Mr J Kreutz has done some illuminating (ha!) calculations on the practical implications of acetylene for motor transport. Here are his conclusions:
"The disadvantages of such acetylene generators are obvious: when leaving Brussels to go on holidays to Nice with an acetylene powered car, the distance to cross is about 1200 km shared in two 600 km legs. Each day the generator must be loaded with 98 kg carbide and 55 litres water. At the end of each day, 114 kg of calcium hydroxide has to be disposed of.
"The acetylene generators and their associated equipments as described in the “Schweizerische Bauzeitung” weigh up to 200 kg or more for providing an autonomy of a merely 160 km. The exploitation cost and efficiency of such a car are at least questionable, specially if it should furthermore carry 4 or 5 persons and their luggage which adds to the overweight of the acetylene generator.
It is clear that only the dire need for fuel in World War II justified the development of such alternative systems, which were also highly successful in France in this period: between 1940 and 1944 tens of patent applications were filed. Before and after the war, such applications are at best scarce for an obvious reason: an empty tank designed for containing 60 litres gasoline is not very heavy; one person can easily carry it. Completely filled it weighs less than 70 kg. These calculation results speak for themselves:"
GASOLINE  ACETYLENE
60 litres gasoline 45.30kgCarbide98 kg
  Water55 kg
Tank 20 kg System 100 kg
Total weight 65.30 kg Total weight 253 kg |
Thus the acetylene system has a weight penalty of 188 kg, or 414 pounds, which is almost a fifth of a ton. That is a crippling disadvantage.
ACETYLENE ENGINES TODAY
In spite of it all, interest in acetylene as a fuel for engines is still alive, for one reason- you can make it out of coal rather than oil. This is the abstract of a paper Evaluation of acetylene as a spark ignition engine fuel by David L Hilden and Russell F Stebar, published in 1979 in the International Journal of Energy Research, Volume 3, Issue 1, pages 59–71.
"In spite of its known shortcomings as a fuel for spark ignition engines, acetylene has been suggested as a possible alternative to petroleum-based fuels since it can be produced from non-petroleum resources (coal, limestone and water). Therefore, acetylene was evaluated in a single-cylinder engine to investigate performance and emission characteristics with special emphasis on lean operation for NOx control. Testing was carried out at constant speed, constant airflow and MBT spark timing. Equivalence ratio and compression ratio were the primary variables.
"The engine operated much leaner when fuelled with acetylene than with gasoline. With acetylene, the engine operated at equivalence ratios as lean as 0·53 and 0·43 for compression ratios of 4 and 6, respectively. However, the operating range was very limited. Knock-induced preignition occurred either with compression ratios above 6 or with mixtures richer than 0.69 equivalence ratio. Both the indicated thermal efficiency and power output were less for acetylene fuelling than for gasoline.
"Acetylene combustion occurred at sufficiently lean equivalence ratios to produce very low NOx and CO emissions. However, when the low NOx levels were achieved hydrocarbon control was not improved over that with gasoline.
"Despite the potential for NOx control demonstrated in this study of acetylene fuelling, difficulties encountered with engine knock and preignition plus well-known safety problems (wide flammability limits and explosive decomposition) associated with acetylene render this fuel impractical for spark ignition engines."
Unfortunately the explosions and general safety problems, as expected, make it unusable as a fuel by itself.
AN ACETYLENE CAR
A Mr Leland Barber claimed to have successfully and very simply converted a car to run on acetylene. The original article was published in March/April 1980, but can be seen at motherearthnews. There is no mention of detonation, changes to to the compression ratio, or alterations in ignition and valve timing. I am skeptical as to whether the claims are genuine.
ACETYLENE TESTS IN JORDAN
A more recent (2009) report on adding acetylene to diesel in a compression-ignition engine, from the Jordan Journal of Mechanical and Industrial Engineering can be found here.
This is the abstract:
"Studies reveal that acetylene gas produced from limestone (CaCO3)is renewable in nature and exhibits similar properties to those of hydrogen. In the present work, experimental investigation has been carried out on a single cylinder, direct injection, and compression ignition engine run on dual fuel mode with diesel as an injected primary fuel and acetylene inducted as secondary gaseous fuel to obtain data on engine performance and exhaust emissions. Fixed quantity of acetylene was aspirated, and readings were taken at various loads. Dual fuel operation resulted in lesser thermal efficiency when compared to neat diesel operation. Acetylene aspiration reduces smoke, soot formation, and exhaust temperature; and increases NOx emission. The emission of carbon monoxide and carbon dioxide was lower under all operating conditions when compared to diesel operation."
Source: "Performance and Emission of Acetylene-Aspirated Diesel Engine"
T Lakshmanana,and G Nagarajan, Volume 3, Number 2, June 2009, pages 125 - 130 ISSN 1995-6665

  
|